About
Hangzhou Yirui Pharmaceutical Technology Co., Ltd.
Hangzhou Yirui Pharmaceutical Technology Co., Ltd. (hereinafter referred to as “Yirui Pharma”) was established in 2018, focusing on the research and development of small molecule innovative targeted drugs. The indications cover autoimmune diseases, diabetic complications, chronic inflammatory diseases including ophthalmic and dermatological diseases, etc. As a Biotech enterprise, after years of development, Yirui Pharma has established a relatively complete R&D system and technology platform, accumulating rich experience and technology in niche areas. With scientific project establishment, low cost, high efficiency, and effective risk control strategies, Yirui Pharma has driven original innovation.
Our Strenghth
Yirui Pharma always regards innovation as its core competitiveness, committed to promoting the drug R&D process through a combination of independent R&D and technology introduction. It emphasizes the integration of basic research and clinical application to ensure that R&D results can be quickly transformed into market products.
Early development
Our team has disease area expertise, particularly on autoimmune diseases, diabetes complications, and hyperinflammation; Our MedChem can do Hit Identification including Structure Biology, virtual screening, and molecular design chemistry; We are doing Lead Optimization by MedChem and by conducting in vitro and in vivo Pharmacology.
Pre-clinical IND
We are working on regulatory Toxicology, formulation, and process development & manufacture.
Clinical Investigation
Our experienced clinical team is capable of running global studies, from medical research, protocol design to clinical operations, focusing on the unmet medical needs, both from China domestically and from the west.
Our focus
unmet medical needs
According to market demand, Yirui Pharma flexibly adjusts its R&D direction, focusing on indications with high clinical value, striving to occupy a favorable position in the market. Through in-depth market research, Yirui Pharma identifies unmet medical needs, providing clear targets and directions for new drug R&D.
Atopic dermatitis
Global Prevalence of 1~3% in adults and 5~20% in children。50% pediatric patients have recurrent AD, it may last for a lifetime
Plaque psoriasis
The global Prevalence is about 2%;and 1% in China. It often complicates other systemic diseases, such psoriatic arthritis, metabolic syndrome. There is no curative treatment.
Inflamatory Bowel Diesease
The incidence rate of UC in Europe /US is ~200/100,000; the incidence rate of CD is ~10/100,000. Pediatric IBD accounts for about 20% of the total number of IBD patients.
Diabetic Retinopathy
Diabetes for >10 years, more than 50% of patients have such lesions Severe vision loss, if not intervened, inevitably leads to blindness
rich experience and technology in niche areas
Cooperative Innovation
Yirui Pharma actively cooperates with domestic and foreign universities, research institutions, pharmaceutical companies, and investment institutions, establishing a multi-faceted cooperation network to jointly promote new drug R&D and market promotion. Through strategic cooperation, Yirui Pharma enhances R&D efficiency and reduces R&D risks.
Investment opportunity
If you are interested in investing Yirui, you are welcome to discuss with us…
Overseas licensing
Yirui’s rich pipeline products are properly protecte by PCT. Yirui can share some of the regional rights
Partnership
As an innovative Biotech Yirui is looking for potential partners in R&D for co-development.
NewCo
Incubation opportunities are available for some of our patented molecules…
Management Team
The core team of Yirui Pharma is composed of former executives from a German drug R&D company and overseas returnees with PhDs, combined with excellent domestic and international management and technical talents, dedicated to building an efficient team with international cutting-edge technology.

Dr. Wang Xiaolu
Founder, Chairman, CSO
PhD from the University of Kiel, Germany, with 16+ year R&D experience in a leading innovative drug company in Europe as SVP; Success in leadership of 20+ innovative drug projects with milestone achievements including market approval; Pioneer experience in establishing preclinical and clinical collaborations between China and EU/US.

Mr. Shen Tao
General Manager
Graduated in clinical medicine, with experiences as a physician in China; 20+ years experiences in pharmaceutical marketing management; success as sale director of GSK China; Former CEO of Shenzhen Leihu Medical Equipment and Device Manufacturing Company

Dr. Kevin Wei
Sr. Vice President, Clinical
Certified Medical Doctor and PhD from the Medical School of University of Birmingham. Over 20 years of clinical drug R&D experience serving as Scientist, Clinical Director, and Vice President of Clinical Medicine in several MNCs.

Dr. Leslie Li
Vice President, Pharmacology
PhD from the University of Miami Miller School of Medicine, USA, with over 20 years of research experience in biology, pharmacology, and translational medicine in innovative companies in US and in China; Success in leadership of 8 IND submissions in US and China.

Dr. Wu Lin
Vice President CMC.
PhD from Nottingham Universtiy UK, with 40 years of experience in biopharmaceutical and small molecule drug delivery and formulation R&D across academic and pharma industry including 20 years at Quotient. Have led more than 80 R&D projects to support clinical trials with a number of products successfully launched in the market.

Dr. Jianfeng Ji
Director, Medicinal Chemistry
PhD from China Pharmaceutical University, with 15+ years of R&D experience in the design, synthesis, screening and optimization of innovative small molecular drugs; Experiences as director of MedChem at innovative companies in China.

Dr. D. Wolff
BD representative
PhD from Berlin Freedom University, with 30+ years of experience in R&D and in business development; successfully made multi-billon deals across the globe

Yan Tang
Financial Director
With 20+ years of experience on finance controlling, treasury, reporting and accounting; Successful IPO experience in both HKEX and SSE STAR Market (Sci-Tech innovation board) for Biotech company; Advanced experience in license in/out, collaboration, equity investment in the field of Biotech.
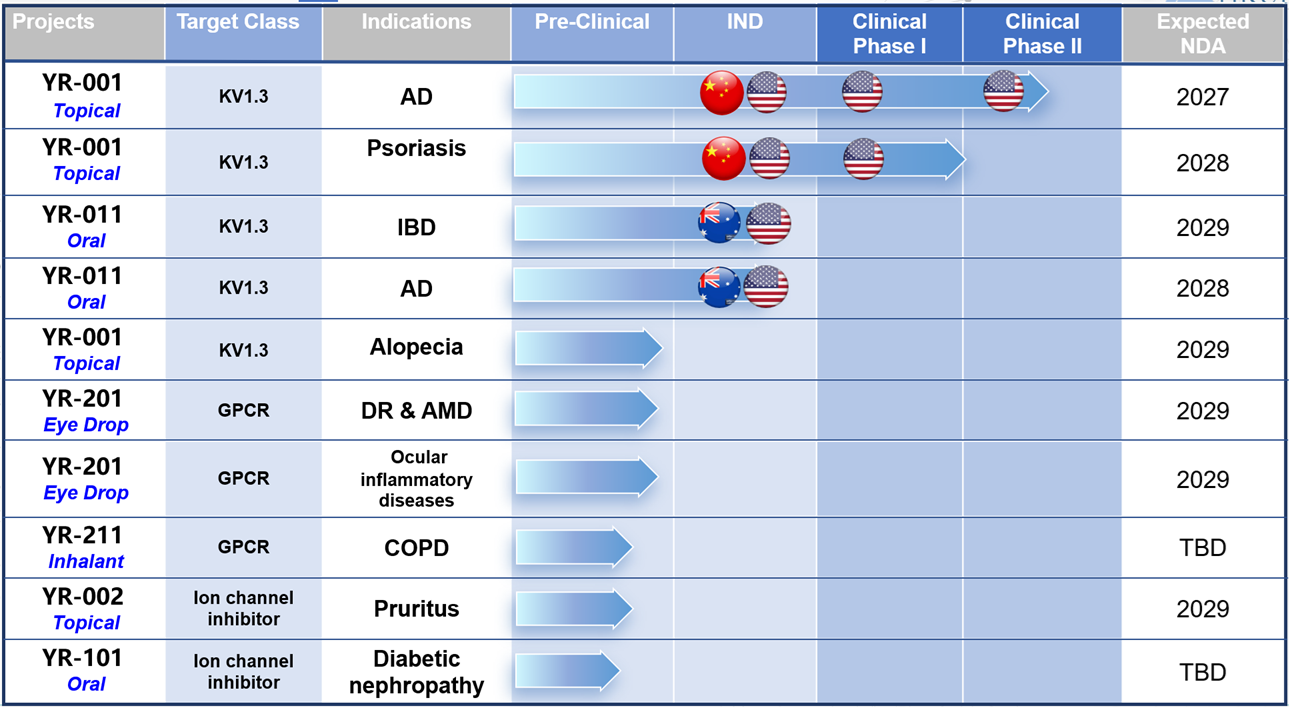
Pipelines and Partners
Yirui’s innovative pipelines are fast moveing forwards…













Get in touch !